Protein Structures
I approached one of my computer science instructors about research and he asked me to do some research of my own on the subject so that I could work with him. And I tell yah, protein structures and functions are some of the most complicated things I've ever seen.
Protein Basics
Well, when I started doing research, I had no clue what a protein even is, and it turns out that there are a couple of other things you need to understand before we can understand protein structures.
There are several characteristics of proteins:
Ligands
Ligand bonding is what occurs when there are stemming connecting atoms to a central metal atom. The molecule that is bound is called the ligand and this molecule can be anything from an oxygen molecule all the way to being as large as a DNA sequence.
Proteins are Flexible
Proteins are flexible, and can change shapes for a couple of reasons.
When the solution that the protein is in, changes pH.
Through ligand bonding (See above).
Amino Acids
The essential building blocks of proteins comes from amino acids. An amino acids is an organic compound with an amine (-NH2) and a Carboxyl (-COOH) functional groups (See fig 1).
A generic amino acid
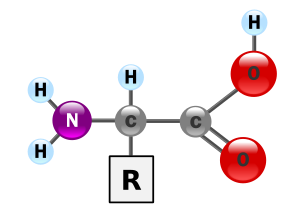
Fig. 1 Source: Wikipedia
Amino acids also have something called a side-chain (R) which is a molecule that is unique depending on the amino acid type. Proteins are made of something like 20 different amino acids which are joined together using
. This is also known as the Primary Structure.
Peptides and Poly-peptides
A poly-peptide is a linear organic polymer consisting of a large number of amino-acid residues bonded together in a chain, forming part of (or the whole of) a protein molecule. In order for a peptide to be able to function as a protein, it needs to be able to form a tertiary structure, this can't be too rigid though due to the demands of the protein.
The Four Levels of Protein Structure
There are four levels of protein structure, and as you could probably guess, they are named as such. Primary Structure, Secondary Structure, Tertiary Structure, and Quaternary Structure.
Primary Structure
As stated in the Amino Acids the primary structure of a protein is the sequence of amino acids, this is determined by the order of the
of the gene encoding it. The primary structure also determines how the protein will fold into higher-level structures.
Secondary Structure
The secondary structure of the protein determines how the proteins moved, and is made of hydrogen bonds and consists of two different forms,
, and
if the carboxyle ends and the nitrogen ends all line up (are on the same ends of the sheet) then this is a parallel
If the carboxyle ends wrap around to meet the nitrogen end, then it is anti-parallel
. This is formed by N-H and C=O groups which are part of the backbone or the main chain. The elements of the secondary structure are folded into the Tertiary Structure.
Tertiary Structure
An alpha helix and a beta sheet
Fig. 1 Source: Bioninja
The Tertiary Structure is consistent of the distant interactions among the secondary structure, this includes hydrogen bonds (again) and Van der Waal interactions. Tertiary structures also include the
, it also includes the
, the di-sulfide bridge only occurs in Cys-stines, and it only occurs on the outside of cells, this is because on the inside of the cell there are anti-oxidants.
The tertiary structure contains the folds of the protein, there are many folds that aren't represented in nature and industry makes use of this to synthesize custom folds for industrial and medical purposes.
Many of the protein's amino acids have unique chemical characteristics that begin to come into play here:
Hydrophobic: The residue chains are insoluble in water, and will clump closer together when submerged in polar molecules in order to minimize contact with water, or other polar side chains.
Hydrophilic: Meaning that the the residue chain is polar, it can create hydrogen bonds with other residues, the backbone, other polar organic molecules, and water. This polar bonding tendency dominates this types' interactions. Some of these molecules can change their charge depending on the pH of their environment.
Amphipathic: This residue has both polar and non-polar components, meaning a mixture of both of the above, this makes these molecules great for interfacing between proteins.
Quaternary Structure
The quaternary structure of a protein is basically the interaction between multiple peptides, they also use hydrogen bonds (again again), and Van der Waal interactions. In a protein, each poly-peptide is a called a sub-unit, and when naming the structure, the proper-confirmation is how many sub-units a protein has.
Sources
Alpha Helix and beta sheet picture
Amino Acid Wikipedia Article
Ligand Wikipedia Article
Protein Structures & Function by: Petsko
Next time I will discuss how DNA is used to create a protein!
Comments
Post a Comment